Health
WHO approves first Mpox vaccine, paving way for increased global access
By Twink Jones Gadama
In a significant move to combat the ongoing mpox outbreaks in Africa and beyond, the World Health Organization (WHO) announced the approval of the MVA-BN vaccine, manufactured by Bavarian Nordic A/S, as the first mpox vaccine to be added to its qualification list.
This milestone decision is expected to substantially improve vaccine availability in affected areas, particularly in Africa, and bolster efforts to reduce infection and control the spread of the disease.
According to WHO Director-General Tedros Adhanom Ghebreyesus, “This first qualification of a vaccine against mpox is an important step in our fight against the disease, in the context of current epidemics in Africa, and in the future.” He emphasized the need to increase the purchase and distribution of vaccines to ensure access to those most at risk.
The MVA-BN vaccine, administered in two doses four weeks apart to adults aged 18 years and older, has demonstrated impressive efficacy rates.
Current statistics show that the vaccine is 76 percent effective after one dose and 82 percent effective after two doses.
WHO Assistant Director-General Yukiko Nakatani noted that the decision to qualify the vaccine will accelerate procurement by international organizations and expedite national regulatory approvals.
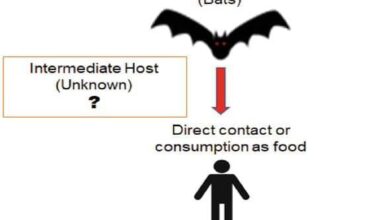
The approval of the MVA-BN vaccine comes at a critical time, as Africa continues to grapple with mpox outbreaks.
The WHO has reported numerous cases and deaths in several countries, highlighting the urgent need for effective prevention and control measures.
By adding the MVA-BN vaccine to its qualification list, the WHO aims to enhance global access to this critical tool, particularly in resource-constrained settings.
The qualification process involves a rigorous evaluation of the vaccine’s safety, efficacy, and quality.
The WHO conducts a thorough assessment of the manufacturer’s facilities, production processes, and quality control measures to ensure compliance with international standards.
While the approval of the MVA-BN vaccine marks a significant milestone, the WHO emphasizes the need to continue collecting data on its safety and effectiveness.
Ongoing monitoring and evaluation will be crucial in ensuring the vaccine’s long-term impact in controlling mpox outbreaks.
In addition to the MVA-BN vaccine, the WHO is working closely with partners to develop and distribute other mpox vaccines, diagnostics, and treatments.
The organization is also providing technical assistance and support to affected countries to strengthen their response to the outbreaks.
As the global health community continues to navigate the challenges posed by mpox, the approval of the MVA-BN vaccine offers a beacon of hope.
With increased access to effective vaccines, countries can better protect their populations and ultimately control the spread of the disease.
The WHO’s approval of the MVA-BN vaccine represents a critical step forward in the fight against mpox.
As the organization works to increase vaccine availability and access, it is essential to continue monitoring the situation and adapting response strategies to address the evolving needs of affected communities.